The Iodine Clock reaction is the official stopping mechanism for the 2016-2017 Junior Chem-E-Car design team. The reaction involves a color change with an Iodine indicator to signal the stopping mechanism, via a light dependent resistor. Through calibration, the reaction time can be determined by the ratio of Potassium Iodide and Sodium Thiosulfate. The correlation between time and ratio is linear; therefore, the Iodine Clock is a simple and effective stopping mechanism for the motor.
The two simultaneous chemical reactions shown below cause a color change from colorless to dark purple with a transient light blue intermediate stage.

Figure 1: Color changes during the iodine clock reaction [Ref. 1]
The first reaction slowly oxidizes iodide ions (I-) into iodine (I2) which has the potential to form a blue triiodide – starch complex. The second reaction rapidly reduces the product (I2) from the first reaction back into its ionic form (I-). Due to the reaction rate difference, only same amount of triiodide exists in the solution which maintains the colorless state of the mixture. When thiosulfate ions (2S2O32−) are exhausted, chemical equilibrium moves to the accumulation and the solution turns from colorless into dark blue. In this calibration process, the concentration of thiosulfate ions acts as the limiting reagent.
During the testing process, our team has to prepare multiple solutions with varying concentrations. We begin by preparing solution A and B (explained in detail below), mixing them, observing and recording the color change. After a large enough spread of data points are recorded, we can generate a calibration curve.
Preparation of solution A
Preparation of solution B
Mixing Solutions and Data Calibration
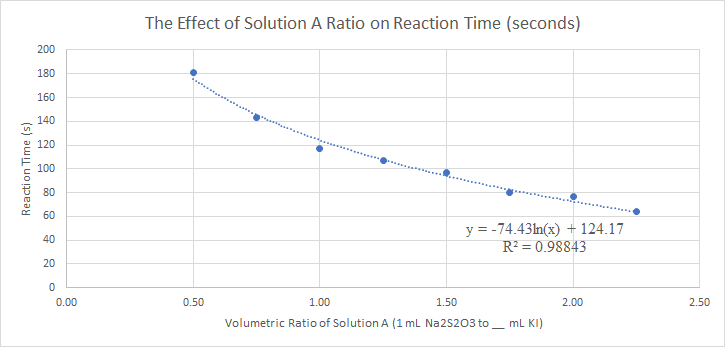
Figure 2: Calibration curve plotting reaction time vs volumetric ratio of solution A to solution B
The Iodine clock reaction displays a linear relationship between reaction time and the ratio between the volumes of Potassium Iodide and Sodium Thiosulfate added. As a result, we can manipulate the volume of our Potassium Iodide solution added to interpolate for a specific stopping time. Our goal is to maintain an error of less than 2% for our calibration curve. The Iodine Clock reaction is a reliable and simple method for timing the motor of the car.